Abstract
Introduction
Approximately 5-10% of patients with chronic lymphocytic lymphoma (CLL) will develop transformation to a more aggressive lymphoma, usually diffuse large B-cell lymphoma (Richter's transformation, RT). The median overall survival after transformation is less than one year. It remains difficult to predict which patients will transform although there is a correlation with poor risk features of CLL, like del17p/TP53 mutation and Notch1 mutations among others. While data emerging from trials of CD19-directed CAR-T cells (CD19CART) in CLL are showing promising results in the relapsed/refractory setting, there appears to be an emergence of RT in some cases even when there is no measurable residual CLL. For instance, in the phase 1 portion of the TRANSCEND CLL 004 trial, in the monotherapy arm with lisocabtagene maraleucel (n=23), 5 RT cases emerged subsequently and 3 of these had no recurrent CLL or MRD conversion to positive [Siddiqi T, et al. ASH 2020]. Four of these RT events were in patients who had progressed on both ibrutinib and venetoclax.
Here we describe patients who developed RT after receiving CD19CART for CLL at City of Hope.
Methods
A retrospective chart review was performed to identify RT emergence and to analyze key factors surrounding the development of RT after CD19CART for CLL at City of Hope. Patient characteristics were assessed including age, sex, prior number of treatments, CLL FISH panel, mutational analysis, time on BTK inhibitor therapy, response to CAR T cell therapy, time to RT after CD19CAR T cell therapy, and outcomes after RT. Pathology samples from RT were assessed for CD19 expression and will be assessed for PDL-1, MYC, SYK, ZAP70, AKT, ERK expression by IHC or flow cytometry.
Results
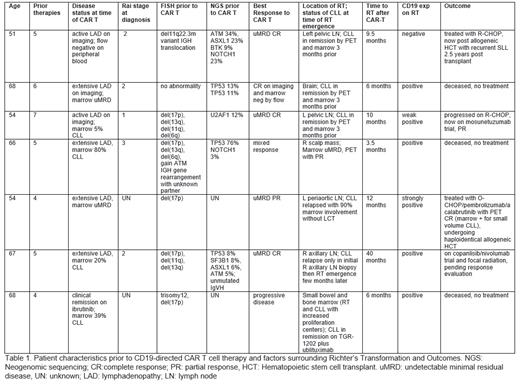
A total of 7 out of 27 patients have been identified who received CD19CART for CLL at City of Hope and subsequently relapsed with RT [Table 1]. The median age at the time of CD19CART was 66 years (range, 54-68) and median number of prior therapies was 5 (range 4-7). All patients had features associated with high risk CLL prior to CD19CART: 5/7 had del17p; 3/7 had TP53 mutations, 2/7 had NOTCH1 mutations, and 1/7 had SF3B1 mutations. Most patients, 6/7, achieved an objective response to CD19CART with 4/7 undetectable minimal residual disease to a level of <10 -4 cells (uMRD4) CRs on imaging and bone marrow examination, and 1 uMRD4 PR. The median time to transformation after administration of CD19CART was 9.5 months (range 3.5-40 months). All patients had received BTK inhibitor therapy prior to CAR T cells, with the median length of treatment being 1 year (5 months - 4 years) and 6/7 had received prior venetoclax as well. Biopsy material at the time of RT indicated 6/7 were positive for CD19 expression by immunohistochemistry or flow cytometry (1 was only weakly positive). PD-L1, MYC, SYK, ZAP70, AKT, ERK expression will be analyzed, and results presented at the meeting.
Of these patients, 3/7 were unable to be treated for RT and died shortly after diagnosis of RT due to frailty, sepsis/respiratory failure/compartment syndrome, and CNS involvement/altered mental status/hypercalcemia/tumor lysis. Two patients achieved CR (one with R-CHOP, one with O-CHOP/pembrolizumab/acalabrutinib) and underwent allogeneic hematopoietic stem cell transplantation - one of which now has relapsed SLL 2.5 years later. Two patients are on clinical trials and are pending response evaluation.
Conclusions
Given the expression of CD19 in the RT pathology of most cases in this series, it appears that a different mechanism of escape or resistance is occurring in these cases. All 7 pts had poor risk features of their CLL before CD19CART like del17p/TP53 mutation, Notch1 mutation and SF3B1 mutation. We are investigating the RT pathology specimens further and will compare these RT cases with other CLL patients we have treated with CD19CART thus far and who have not relapsed/progressed with RT in order to examine the differences in treatment history, cytogenetic features, proliferative/accelerated nature of CLL at baseline, and PDL1 expression before and after CAR T cell therapy. Improved treatment combinations are needed in high risk, multiply relapsed CLL patients to prevent emergence of RT despite excellent responses of the CLL itself.
Danilov: Gilead Sciences: Research Funding; Pharmacyclics: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; TG Therapeutics: Consultancy, Research Funding; Takeda Oncology: Research Funding; Genentech: Consultancy, Honoraria, Research Funding; SecuraBio: Research Funding; Bayer Oncology: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; Bristol-Meyers-Squibb: Honoraria, Research Funding; Rigel Pharm: Honoraria. Siddiqi: Janssen: Speakers Bureau; Oncternal: Research Funding; Pharmacyclics LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Research Funding; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
CD19 CAR T products used in clinical trials for relapsed/refractory chronic lymphocytic leukemia

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal